noble gas configuration for fe|Electronic Configuration of Iron : Clark How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh . The KEEP is a conditioning method for fighting fowl that is patterned after the latest studies of human athletic competition. The principle behind conditioning birds is “carbohydrate loading.” To .
noble gas configuration for fe,Electron Configuration for Iron (Fe, Fe2+, and Fe3+) Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period .

In order to write the Calcium electron configuration we first need to know the .

In order to write the Calcium electron configuration we first need to know the .In order to write the Na electron configuration we first need to know the .
How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .
In order to write the Mg electron configuration we first need to know the .Iron (Fe, Fe 2+, Fe 3+) Read my article in Science Education based on my .When we write the configuration we'll put all 15 electrons in orbitals around the .Because the third energy level has eight electrons and is therefore full (3s 2 3p 6) .How to Write the Electron Configuration for Oxygen. Oxygen is the eighth element . Mar 23, 2023
A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. .
Electronic Configuration of Iron. The chemical element iron has the atomic number 26 and the symbol Fe (from Latin: Ferrum). It’s a transition metal from group 8 of the periodic table’s first transition series.Nobel Gas Configuration. Electron Configuration of Transition Metals and Inner Transition Metals. Exceptions. Summary. Problems. Answers. Contributors. Learning Objectives. Using the Aufbau Principle, the Pauli .The arrangement of electrons in iron in specific rules in different orbits and orbitals is called the electron configuration of iron. The electron configuration of iron is [ Ar] 3d 6 4s 2 , .Finding Noble Gas Configuration. A short cut method of writing configurations. Since noble gases are “special” – reference all configurations against the PREVIOUS noble .
Remember, the noble gases are the elements that are nearly non-reactive, partially due to the fact that the outermost energy level electron configuration was full ( .The noble gases (historically the inert gases, sometimes referred to as aerogens) . As a result of a full shell, the noble gases can be used in conjunction with the electron configuration notation to form the noble gas notation. To do this, the nearest noble gas that precedes the element in question is written first, and then the electron . So that's the electron configuration for silicon. Now, we can write it out using noble gas notation. And compare, so, the noble gas immediately preceding silicon, if we go up a row and then move over, we see that it's neon. So we write neon in brackets. . Put the brackets around the electron configuration of the last noble gas, group 18 element, before nitrogen. Note that helium (He) is the noble gas preceding nitrogen. Continue with the electron . Start with the electron configuration of the neutral atom. It is sufficient to use the noble gas configuration. Fe: [Ar] 4s 2 3d 6. Then, remove (or add) electrons to reflect the oxidation state of the metal. Remember to remove electrons from the orbital with the highest principal quantum number first! Fe(III) has lost 3 electrons relative to .This method of writing configurations is called the noble gas notation, in which the noble gas in the period above the element that is being analyzed is used to denote the subshells that element has filled and after which the valence electrons (electrons filling orbitals in the outer most shells) are written. . The element is iron, Fe. b) 1s .
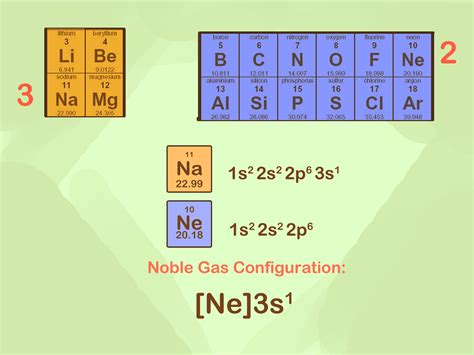
A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] [ Ne] for the 1s22s22p6 1 s 2 2 s 2 2 p 6 part of the configuration. Sodium's noble gas configuration becomes [Ne] 3s1 .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a . Remember that when we write electron configuration codes using noble gas configurations we use the previous noble gas. In writing the electron configuration code for Fe or Fe 2+, we would use the symbol [Ar] to substitute for 1s 2 2s 2 2p 6 3s 2 3p 6.This page titled 5.20: Noble Gas Configuration is shared under a CK-12 license and was authored, remixed, and/or curated by CK-12 Foundation via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. A noble gas configuration of an atom consists of the elemental .
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. . Electron configuration of Iron (Fe) [Ar] 3d 6 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2: 2, 8, 14, 2: 27: Electron configuration of Cobalt (Co) [Ar] 3d 7 4s 2: 1s 2 2s 2 2p 6 3s 2 3p .Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 . Look up the electron configuration of any element using this handy chart. The chart lists elements in order of atomic number. Menu. . The common shorthand notation is to refer to the noble gas core, rather .noble gas configuration for feUsing abbreviated electron configuration (or noble gas configuration) to identify valence electrons; . (n-1) d orbital. For example, the electron configuration of iron is Fe is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the . A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] for the 1 s2 2 s2 2 p6 part of the configuration. Sodium’s noble gas configuration becomes [Ne]3 s1.A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] for the 1 s2 2 s2 2 p6 part of the configuration. Sodium’s noble gas configuration becomes [Ne]3 s1.noble gas configuration for fe Electronic Configuration of Iron The noble gas notation for Iron (Fe) is [Ar]4s2 3d6. . What is noble-gas notation and why is it used to write electron configuration? The noble gas notation is a notation formed as a result of .
PROBLEM 3.1.12 3.1. 12. In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, Co 2+ and Co 3+. Write the electron structure of the two cations. Answer.
Noble Gas Configurations. Sodium, element number eleven, is the first element in the third period of the periodic table. Its electron configuration is 1s 2 2s 2 2p 6 3s 1. The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the .
noble gas configuration for fe|Electronic Configuration of Iron
PH0 · Noble Gas Configuration and Configuration of Ions
PH1 · Noble Gas Configuration
PH2 · High School Chemistry/Electron Configurations
PH3 · Electronic Configuration of Iron
PH4 · Electron Configuration for Iron (Fe, Fe2+, and Fe3+)
PH5 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · 7.3: Electron Configurations of Atoms
PH8 · 6.7: Electronic Configurations
PH9 · 5.20: Noble Gas Configuration
PH10 · 3.1: Electron Configurations